Objective: To compare the concordance of the Ultrahuman cycle tracking (CT) algorithm behind the ‘Cycle and Ovulation’ power plug to the calendar method and to urine-based ovulation prediction kit (OPK).
The menstrual cycle forms the foundational basis of a large part of a woman’s life, with its onset or cessation marking dramatic physiological changes. It is also inextricably entwined with the estimation of the fertility period, and together these phenomena have important ramifications for her health, lifestyle, and career. While cycle tracking (CT) is based on empowering a woman with the information needed to decide whether to utilize or avoid fertility windows, it also becomes important for understanding changes during the menopausal transition and decoding variations in her body which may also signal onset of diseases like PCOS, endometriosis or even cancers.
Over centuries CT could only be done via counting the days per what is called as the calendar method (also called rhythm method) in addition to tracking changes in vaginal mucus or saliva1. Around the early 20th century, with the widespread use of thermometers, a biphasic shift in women’s basal body temperature (BBT) was noticed during the menstrual cycle. This concept was further refined to develop a temperature-based method of fertility prediction which tracks a dip followed by a subsequent and sustained rise in body temperature triggered by ovulation1, 2. With advances in endocrinology, the orchestrated action of follicle-stimulating hormone (FSH), estrogen, luteinizing hormone (LH) and progesterone was understood better1, 3. LH surge became the definitive marker for ovulation or peak fertility events, with at-home testing available (also called Ovulation Prediction Kits, OPK) since the late 1980s4.
It is important to note that the gold standard for assessing follicle maturity and ovulation remains the transvaginal ultrasound procedure5. However, given the invasive nature and dependence on clinical supervision, the use of this procedure remains limited to in vitro fertilization regimes and special medical cases. The overwhelming majority of women globally either rely on the calendar method or OPKs. These techniques are used not only for family planning but also to determine train capacity in female athletes, monitor interactions with medications, and map hormonal impacts on metabolism, cardiac health, and cognitive function in women with clinical conditions.
Recently, the fact that ‘female’ hormones that govern a woman’s menstrual cycle also impact other organ systems is being studied in depth. To date evidence points to the fact that women undergo cyclical changes in heart rate, heart rate variability, levels of water retention, metabolism, hunger/satiety, olfactory acuity etc., largely in sync with their menstrual cycle6, 7. Among these, distal (away from the core thoracic area) heart rate has been demonstrated to undergo distinct phase shifts throughout the cycle and has been shown to have strong association with LH surge and progesterone plateaus in the luteal phase. Coupled with continuous data streams available from wearable devices this offers a more nuanced and personalized approach to CT8, 9. Multiplexing temperature and heart rate (which can be passively acquired through wearable technology) can serve as convenient proxies to track phases of the menstrual cycle and fertility windows. This approach was first verified by Goodale et al., 2019 using a wrist form factor wearable10. Evidence of a similar and multivariate approach for fertility prediction validated with hormonal data is sparse. To fill this gap we undertook a pilot study to validate the Ultrahuman CT algorithm and with commonly used Inito OPK.
For ideation of this feature, cycle data of existing Ultrahuman Ring AIR female user base (complete or fragmented) was initially collected, totalling over 15600 cycles. The initial cadence of heart rate (HR), heart rate variability (HRV) and skin temperature was mapped across a rolling 30 day period to identify the trends and variability. Attributes such as BMI, age, consistency of Ring use (totally 4055 cycles) were taken into consideration while refining the algorithm.
Validation of the algorithm was carried out among a group of 22 female volunteers with an age range of 23-42 years, reporting cycle lengths between 24 and 40 days. These women were not on any medication or contraception. They were directed to collect hormonal data from urine metabolites of four main hormones - estrogen (estrone-3-glucuronide or E3G), luteinizing hormone (LH), follicle stimulating hormone (FSH) and progesterone (pregnanediol glucuronide) for three successive cycles using a clinically validated, FDA-certified class 1 device - the Inito home fertility monitoring system (Inito)11. Informed and signed consent was collected from the participants for a) collection of their Ultrahuman Ring AIR biomarkers, and b) sharing of their Inito OPK output with Ultrahuman analysts. Declarative menstruation start and stop dates were collected from all participants throughout the study period.
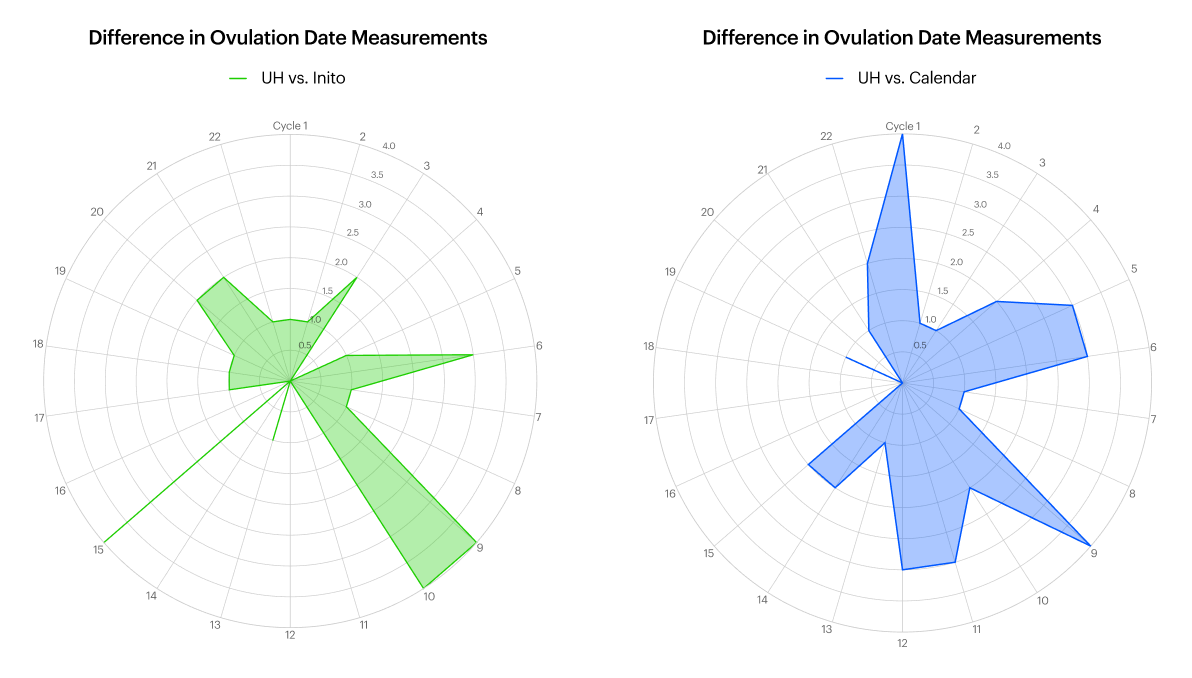
Inito-predicted fertility dates were compared to Ultrahuman algorithm predicted dates, which were in turn compared to the calendar method. Descriptive statistics are only reported. Peak fertility dates corresponding to the LH surge were considered for analysis. A sub-group of ovulation confirmations were also compared when available. The calendar-method cycles were captured by the “mark start of menstrual phase” mode on the Ultrahuman application.Data analysis was performed by a team entirely removed from the participant pool.
Modern day insights into women’s health can benefit a great deal from continuous data streams provided by at-home and wearable devices12. In the short term, the insights can help optimize training by identifying peak performance windows or certain phases that may have minimal difference on the choice of fitness regime on an individual’s body. In the long term, continuous tracking allows for the detection of cycle irregularities or anomalies, offering an opportunity to catch early signs of conditions like PCOS, thyroid issues, or other hormonal imbalances.
Several life-altering physiological events, which cause large changes in metabolism, mental health and fitness, are increasingly being found to meld into each other rather than have discrete start and end points. For example, a healthy woman in her 30s may consider based on her family history that her fertility reserve would last another ten years. However, modern day stressors and diet quality may change her physiology substantially to advance her transition into perimenopause. Continuous cycle tracking can pick up early trends faster not just in terms of cycle length but also in baseline HR, HRV and sleep. Currently women health support is divided rather arbitrarily into fertile/pregnancy and menopause transition stages. Different apps and devices provide support for one or the other, but devices such as the Ultrahuman Ring AIR can provide the continuous bridge data to help navigate life stages.
To register cycle mid-point we chose to monitor an event that is tracked by several applications, namely the LH surge. This hormonal event is followed by release of the egg and is usually central to planning conception or contraception as per needs. LH surges become more sporadic with aging as well as in women with irregular periods. LH surge occurs across a multi-hour window and can span over datelines (12 midnight). Therefore, the recorded 1-day difference between Ultrahuman algorithm and hormonal testing could actually be tracking the same event rather than another at a delay of a day. The calendar method expectedly did have more deviations from the biomarker based method since it is a rigid algorithm without room for changes in cycle length. In older participants and those with irregular cycles, there was less overlap in LH surge detection due to several deviations in continuous biomarkers and multiple small spikes of LH hormone. This is in concordance with known lower prediction overlaps for irregular menstruators13. Taken together, women with consistent cycles in this pilot were able to determine that the Ultrahuman algorithm is reasonably accurate in tracking LH surge as compared to at-home OPK approach.
While easy to use and near continuous, it is to be acknowledged that physiological readouts can be impacted by infection and other disease conditions and hence their accuracy can be compromised2. Severe viral infections or chronic metabolic conditions have been known to shift cycles and cause hormonal imbalance14, 15. Hence non-invasive tracking can’t be a stand alone proxy for conception or contraception goals. Ultrahuman’s approach to women's health is to be a partner through various stages of their health journey. This ‘Cycle and Ovulation’ power plug will be released without a subscription fee globally, integrating the tracking device and the algorithm on the same platform.
Reach out to partnerships@ultrahuman.com for commercial queries and science@ultrahuman.com for scientific queries.
1. Su, H. W., Yi, Y. C., Wei, T. Y., Chang, T. C., & Cheng, C. M. (2017). Detection of ovulation, a review of currently available methods. Bioengineering & translational medicine, 2(3), 238-246. PMID: 29313033 PMCID: PMC5689497 DOI: 10.1002/btm2.10058
2. Baker, F. C., Siboza, F., & Fuller, A. (2020). Temperature regulation in women: effects of the menstrual cycle. Temperature, 7(3), 226-262. PMID: 33123618 PMCID: PMC7575238 DOI: 10.1080/23328940.2020.1735927
3. Hoff, J. D., Quigley, M. E., & Yen, S. S. (1983). Hormonal dynamics at midcycle: a reevaluation. The Journal of Clinical Endocrinology & Metabolism, 57(4), 792-796. PMID: 6411753 DOI: 10.1210/jcem-57-4-792
4. Scolaro, K. L., Lloyd, K. B., & Helms, K. L. (2008). Devices for home evaluation of women’s health concerns. American Journal of Health-System Pharmacy, 65(4), 299-314. PMID: 18238767 DOI: 10.2146/ajhp060565
5. Vermesh, M., Kletzky, O. A., Davajan, V., & Israel, R. (1987). Monitoring techniques to predict and detect ovulation. Fertility and sterility, 47(2), 259-264. PMID: 3817171
6. Draper, C. F., Duisters, K., Weger, B., Chakrabarti, A., Harms, A. C., Brennan, L., ... & Van der Greef, J. (2018). Menstrual cycle rhythmicity: metabolic patterns in healthy women. Scientific reports, 8(1), 14568. PMID: 30275458 PMCID: PMC6167362 DOI: 10.1038/s41598-018-32647-0
7. Farage, M. A., Neill, S., & MacLean, A. B. (2009). Physiological changes associated with the menstrual cycle: a review. Obstetrical & gynecological survey, 64(1), 58-72. PMID: 19099613 DOI: 10.1097/OGX.0b013e3181932a37
8. Alzueta, E., Gombert-Labedens, M., Javitz, H., Yuksel, D., Perez-Amparan, E., Camacho, L., ... & Baker, F. C. (2024). Menstrual Cycle Variations in Wearable-Detected Finger Temperature and Heart Rate, But Not in Sleep Metrics, in Young and Midlife Individuals. Journal of biological rhythms, 39(5), 395-412. PMID: 39108015 PMCID: PMC11416332 DOI: 10.1177/07487304241265018
9. Sides, K., Kilungeja, G., Tapia, M., Kreidl, P., Brinkmann, B. H., & Nasseri, M. (2023). Analyzing physiological signals recorded with a wearable sensor across the menstrual cycle using circular statistics. Frontiers in Network Physiology, 3, 1227228. PMID: 37928057 PMCID: PMC10621043 DOI: 10.3389/fnetp.2023.1227228
10. Goodale, B. M., Shilaih, M., Falco, L., Dammeier, F., Hamvas, G., & Leeners, B. (2019). Wearable sensors reveal menses-driven changes in physiology and enable prediction of the fertile window: observational study. Journal of medical Internet research, 21(4), e13404. PMID: 30998226 PMCID: PMC6495289 DOI: 10.2196/13404
11. Pattnaik, S., Das, D., & Venkatesan, V. A. (2023). Validation of urinary reproductive hormone measurements using a novel smartphone connected reader. Scientific Reports, 13(1), 9227. PMID: 37286704 PMCID: PMC10247788 DOI: 10.1038/s41598-023-36539-w
12. Lyzwinski, L., Elgendi, M., & Menon, C. (2024). Innovative approaches to menstruation and fertility tracking using wearable reproductive health technology: systematic review. Journal of Medical Internet Research, 26, e45139. PMID: 38358798 PMCID: PMC10905339 DOI: 10.2196/45139
13. Yu, J. L., Su, Y. F., Zhang, C., Jin, L., Lin, X. H., Chen, L. T., ... & Wu, Y. T. (2022). Tracking of menstrual cycles and prediction of the fertile window via measurements of basal body temperature and heart rate as well as machine-learning algorithms. Reproductive Biology and Endocrinology, 20(1), 118. PMID: 35964035 PMCID: PMC9375297 DOI: 10.1186/s12958-022-00993-4
14. Vigil, P., Lyon, C., Flores, B., Rioseco, H., & Serrano, F. (2017). Ovulation, a sign of health. The Linacre Quarterly, 84(4), 343-355. PMID: 29255329 PMCID: PMC5730019 DOI: 10.1080/00243639.2017.1394053
15. Monroe, A. (2007). Menstruation, menopause, and HIV. Bulletin of Experimental Treatments for AIDS, 19(2), 39-44. PMID: 17489115